Why use software to conduct internal GMP area audits?
Andy Pritchard | December 7, 2021 | 4 min read
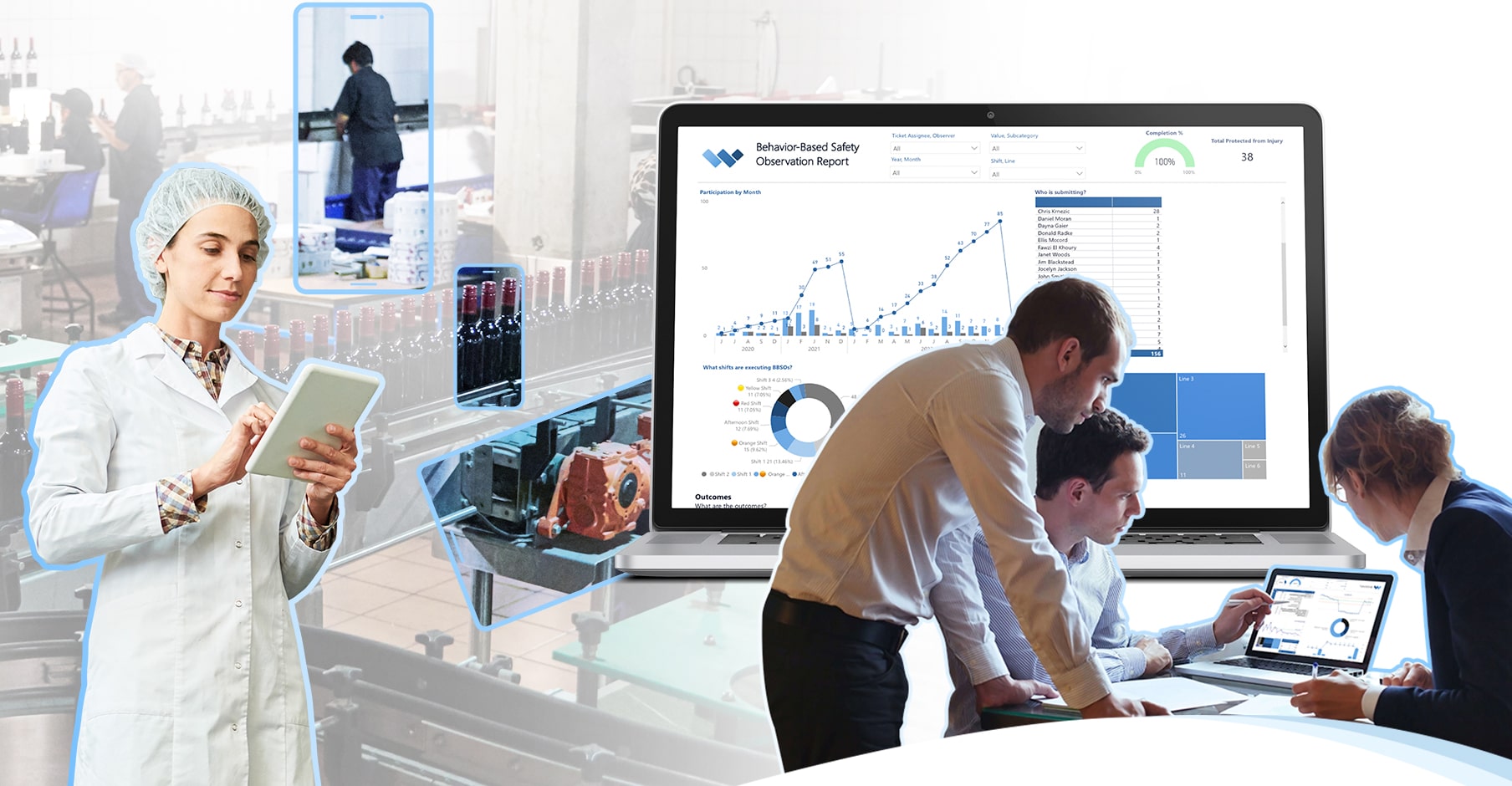
If you are managing a robust GMP compliance program, you are probably mandating pre-production checks daily or per batch, daily hygiene audits, weekly PRP GMP Audits, semi-annually HACCP Audits and annual Systems audits.
Capturing data for all of these audits using paper forms and manual spreadsheet inputs is a huge drain on resources for your organization. Paper forms present many opportunities for mistakes and wasted time, which could be the difference between defective product leaving the facility or not.
There are many advantages to digitizing your GMP audits including increased efficiency, capturing richer data and real-time, automated reporting.
Here are just a few examples of how software can optimize GMP audits.
Efficient Data Capture
Digital forms are immediately accessible. You can set up the form to guide stakeholders through the process so that they get it done right - the first time and every time thereafter.
Automate Scoring & Escalation
Provide feedback to users in real-time. Turn an audit into a live risk assessment so they can not only ensure GMP processes are compliant, but they can evaluate issues and make guided corrective actions immediately.
Capture Richer Data
Paper forms only offer so much space. Best practices with paper forms is to limit fields so as not to confuse staff and ensure efficiency. With digital forms, you can reveal hidden form fields when necessary and have the best of both worlds - rich data and an efficient user experience.
Scheduling
A consistent schedule of checks, inspections and audits is imperative for GMP compliance. However, whether it’s on a manufacturing floor or construction site, if it's not scheduled, it's not going to happen.
Real-time Reporting
Inspections and audits completed with paper forms are likely to stay at the machine or site until they are submitted, which could take hours, days or even weeks, which opens a greater risk of defective products leaving the facility and becoming regulatory compliance issues.
Paperless
It’s 2021. It should be the goal of all organizations to be as paperless as possible.







Marks

Diageo

Niagara Bottling

Walmart

PepsiCo logo

McDonald's

Unilever

Monin

Hello Fresh

Rise Baking

Rockwool

Canadian Tire

SportChek

Greyston Bakery

Bell

Husqvarna

Home Hardware