Workflow Tickets
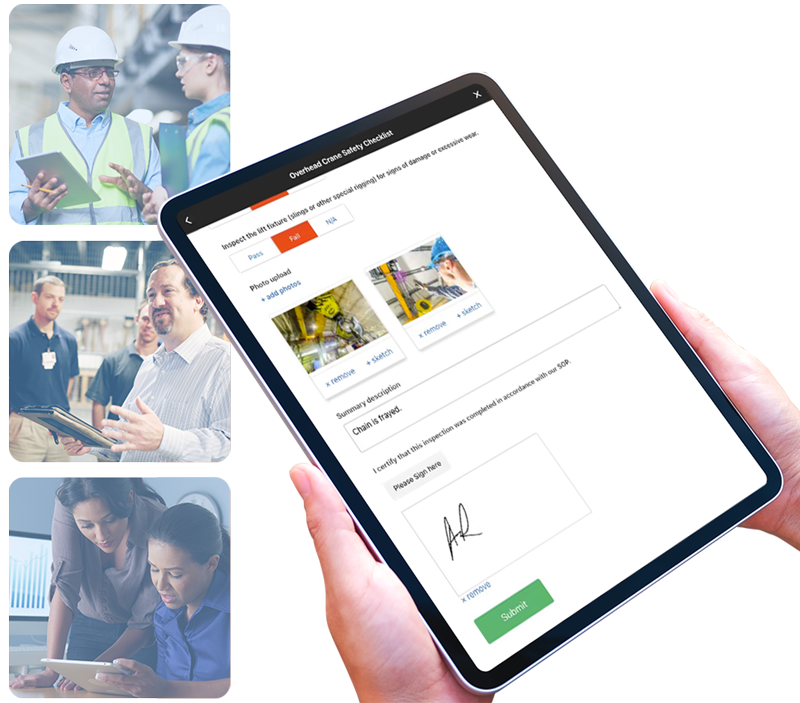
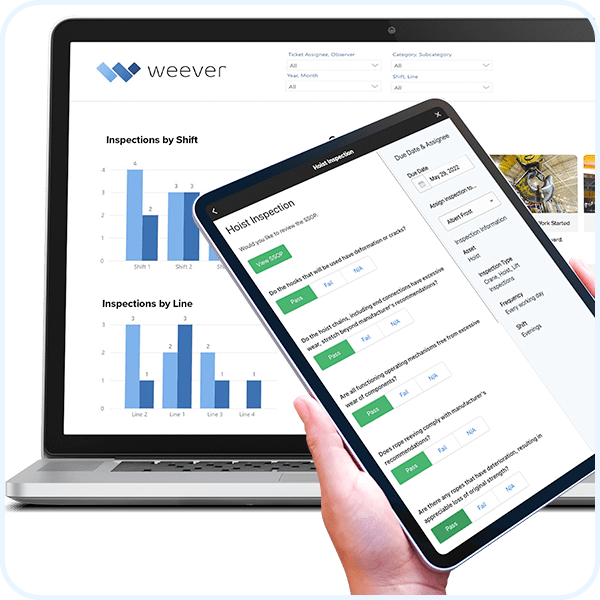
Crane, Lift and Hoist Inspections
Schedule and provide instructions to ensure crane, lift and hoist inspections are done right every time.
Read MoreLift Inspections
Create and manage schedules. Provide real-time, on-demand instructions to ensure compliance.
Read MoreHoist Inspections
Schedule and provide instructions. Capture rich data to ensure safety.
Read MoreBest Practices for GMP Audit Checklist
Home / Zero Blog / Best Practices for GMP Audit Checklist Best Practices for GMP Audit Checklist Andy Pritchard | December 5, 2021 | 4 min read TABLE of contents What does a standard Operational Audit Schedule for a typical food company look like? If harnessed correctly, GMP audits can have a massive…
Read MoreWhy use software to conduct internal GMP area audits?
Why use software to conduct internal GMP area audits? Andy Pritchard | December 7, 2021 | 4 min read If you are managing a robust GMP compliance program, you are probably mandating pre-production checks daily or per batch, daily hygiene audits, weekly PRP GMP Audits, semi-annually HACCP Audits and annual Systems audits. Capturing data for…
Read MoreGMP Operational Audit Schedule for a Food Company
GMP Operational Audit Schedule for a Food Company Andy Pritchard | Dec 7, 2021 | 5 min read What does a standard Operational Audit Schedule for a typical food company look like? Organizations involved in the manufacturing and distribution of food are required by law to audit their processes and equipment to ensure basic…
Read MoreAnnouncing a new collaboration between Fiix and Weever
A new partnership announced today between Fiix Inc. and Weever makes it easier than ever for companies to embrace the power of Industry 4.0 technology to connect maintenance and operations, streamline processes, and use real-time data to bolster the health and performance of both assets and staff.
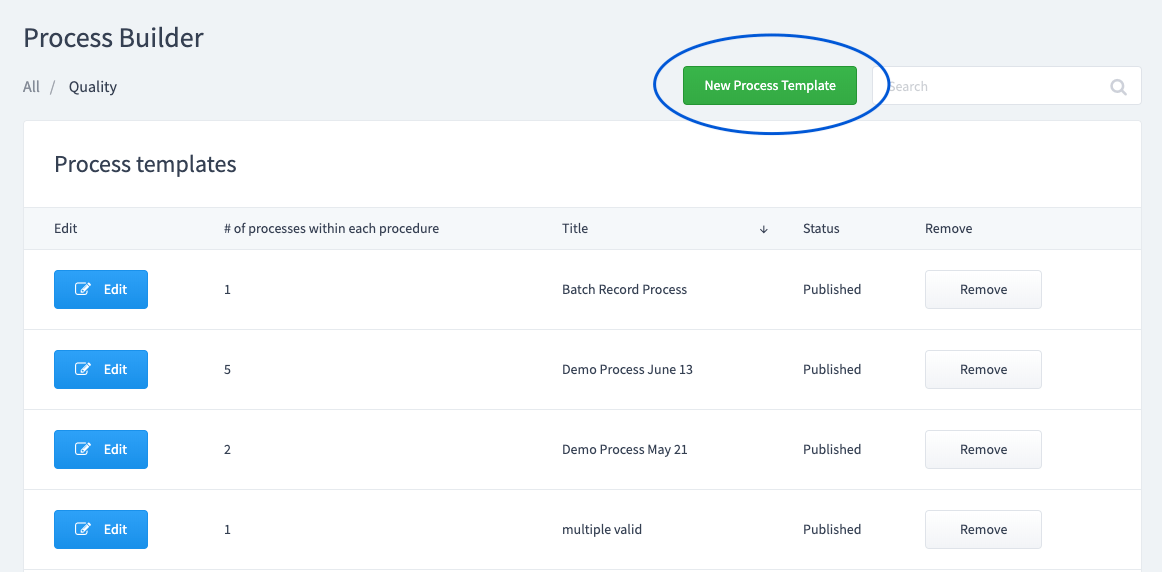
Read MoreHow to build a Process Template with Weever
Process Manager is used to manage all facets of Electronic Batch Record management by food, beverage and drug manufacturers. A major requirement of FDA and CFIA regulations is to determine the “Readiness for commercial manufacturing” and the “conformation to application” of the manufacturers. In simple terms, this basically translates to (1) are you set up…
Read More