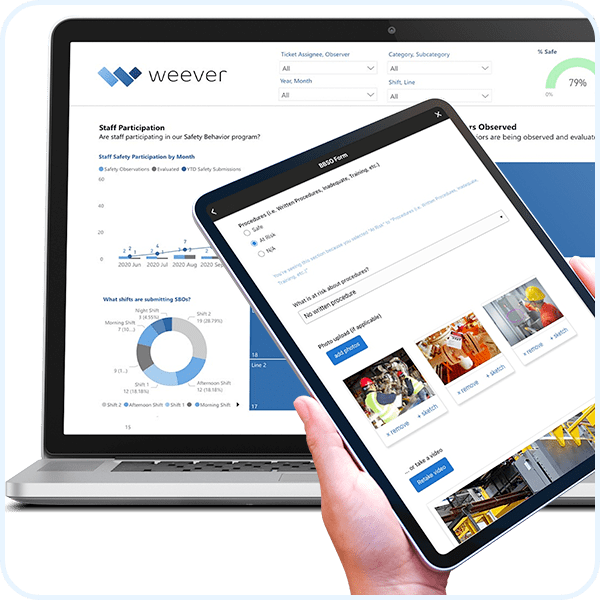
Automate Compliance
For regulated industries, such as food, CPG and pharmaceutical, where data integrity, security and compliance are required, Weever Process ensures zero process shortcuts occur, changes to records are tracked, and auditors are satisfied.










Marks

Diageo

Niagara Bottling

Walmart

PepsiCo logo

McDonald's

Unilever

Monin

Hello Fresh

Rise Baking

Rockwool

Canadian Tire

SportChek

Greyston Bakery

Bell

Husqvarna

Home Hardware
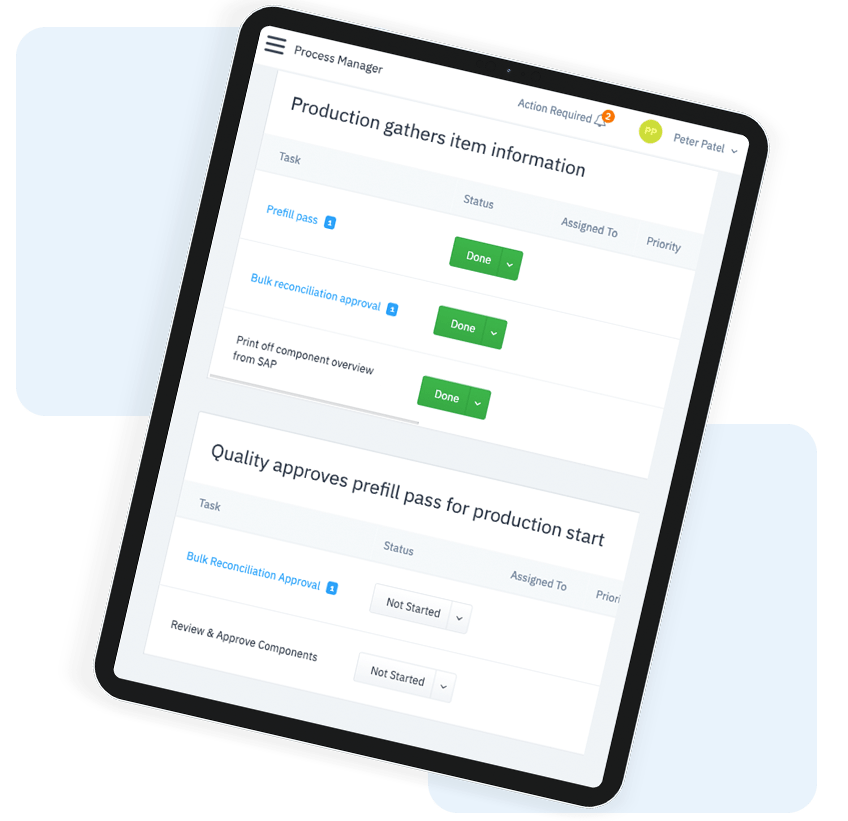
Intuitive Task Completion
Staff access a digital task list that includes instructions and dynamic inputs, which save time and ensure tasks are completed correctly the first time and every time.
Effortless Compliance
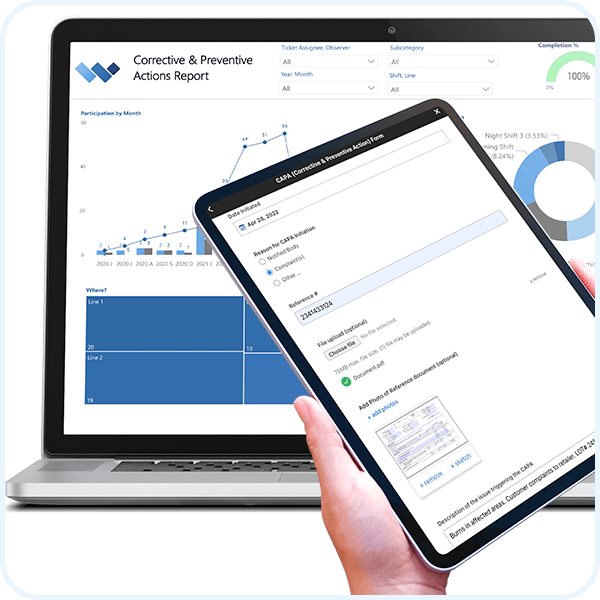
Includes 30+ features to comply with FDA 21 CFR Part 11, EU Annex and Health Canada.
Insight-Driven Dashboards
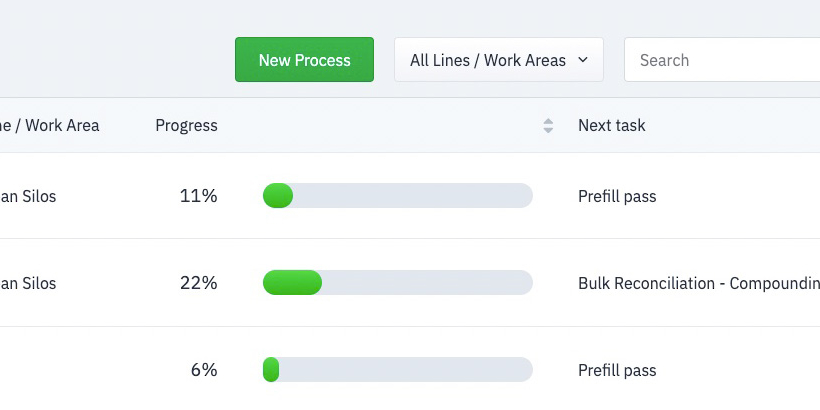
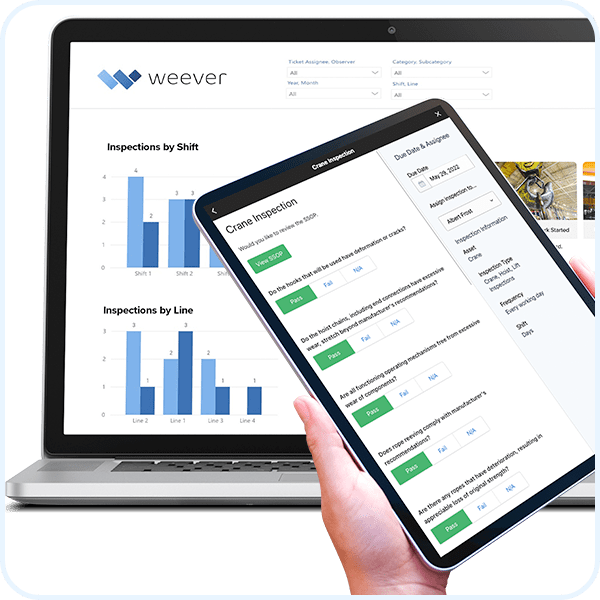
Understand everything when it happens with automatically updated, stunning and insight-driven reporting dashboards.
Key Features
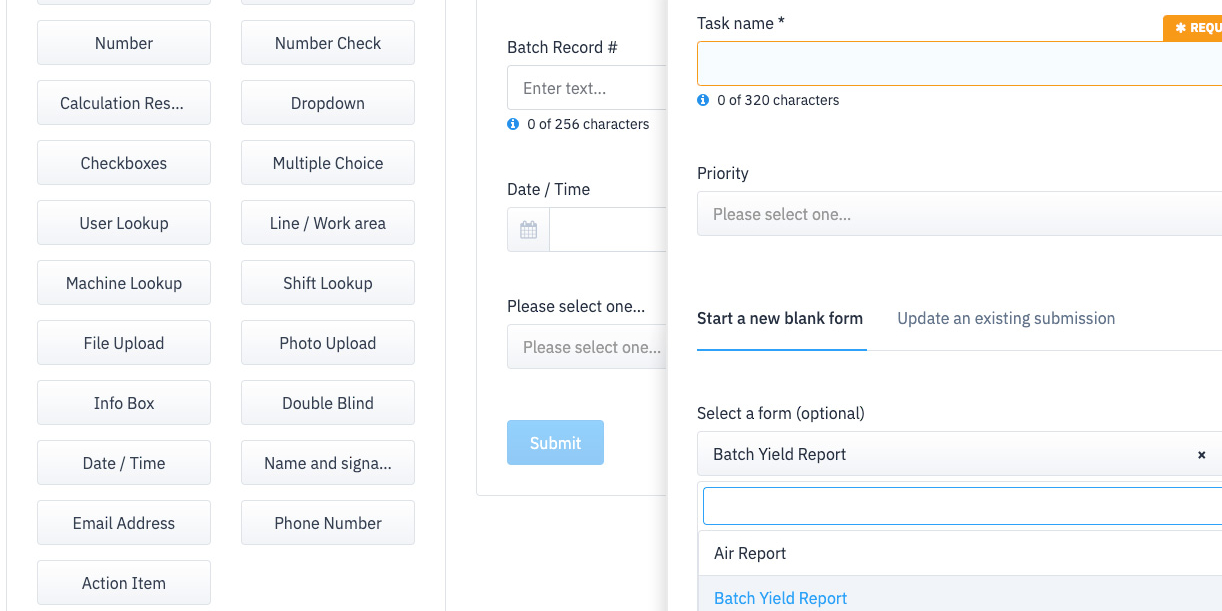
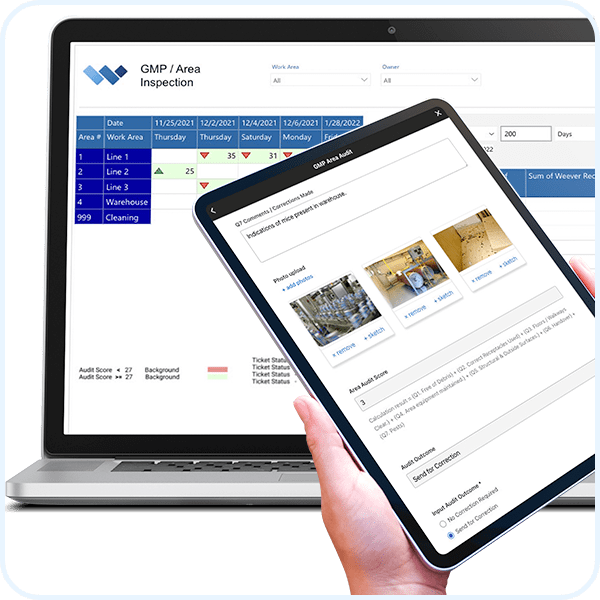
Easily build and adapt your process templates to suit your requirements. Use templates or start from scratch.
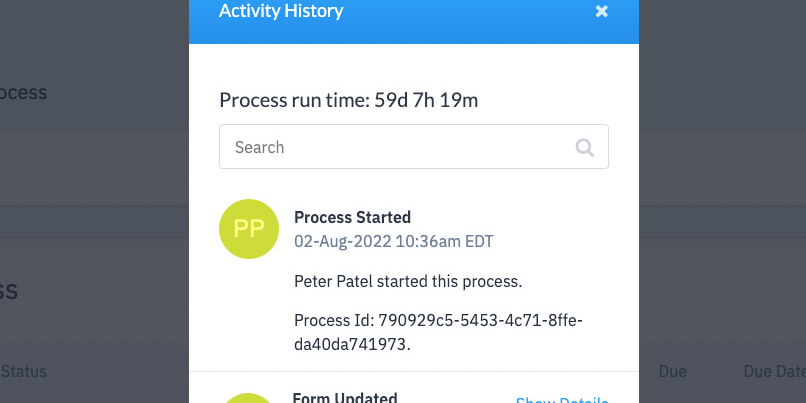
All interactions within the platform are tracked so you can rest assured that you are compliant to FDA audits.
Staff pick it up and go on virtually any device without a lengthy on-boarding process.
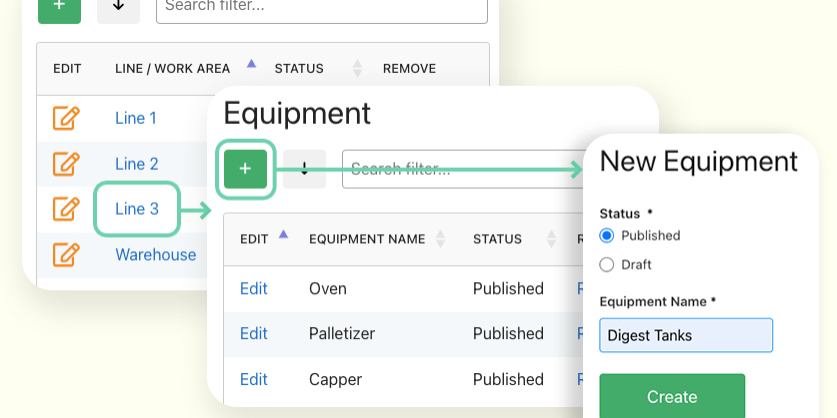
Create and manage lists of common elements at your facility to standardize reports.
Automatically alert supervisors when there is an issue with a process. Collaborate in real-time within the app to determine next steps.
case study video
Martin SC Saves $70K per year with Weever Process
EXPLORE WEEVER Process in action
Weever is universally useful for all your operations management requirements.
- All Solutions
- Compliance
- Continuous Improvement
- Maintenance
- Quality
- Safety
- Skills Development
- Total Productive Maintenance
- Warehouse

100K+
Global Users
MILLIONS
of jobs completed
11
Languages
"The Weever suite is easy to understand and the support from the team has been amazing!"
Laura Curtis - Operational System and Process Manager, HelloFresh
"Weever saves me time, makes data visible and drives results!"
DENAIR M. - Training Manager
"Weever has changed how I run our business."
Ingrid K. - Plant Director
"Weever is extremely easy to use and simple to manage."
NICKI V - CI Engineer
"Weever gives me immediate insights into my business."
COLIN H. - Operational Excellence Manager
"The Weever team are extremely helpful and are always on hand to help with any questions or queries we may have."
Mel Cadle - Op Ex Lead Process Engineer, HelloFresh



SCHEDULE A DEMO
Take a Guided Tour.
In just 30 minutes you will learn about customizing and using forms, workflow automation, reviewing reports, and sharing data with other business systems.
In just 30 minutes you will learn about customizing and using forms, workflow automation, reviewing reports, and sharing data with other business systems.