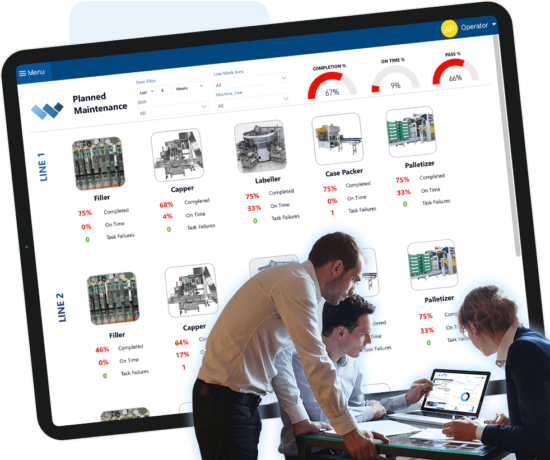
Compliance Management
Don’t leave compliance up to chance. Compliance includes practices that must be adhered to in order to stay in business. Regulations can include FDA, HACCP, OSHA, EPA and other regional, national, or international standards. The risk of non-compliance can be devastating, including loss of business, lawsuits, and safety incidents. Weever ensures compliance by automating data capture, workflow and reporting requirements so the work gets done correctly, the first time and every time.
The Costs of Non-Compliance for Food Manufacturers
The cost of non-compliance for FDA violations can vary depending on several factors, including the nature and severity of the violation, the scale of the operation, and the history of compliance of the manufacturer. Some potential costs associated with FDA violations for food manufacturers may include:
Read MoreHow to increase Employee Engagement by focusing on Ease and Usefulness
According to numerous studies Continuous Improvement program participation can be predicted by the amount of intention employees have to participate, which is inflences by Ease and Usefulness of participation.
Read MoreWhat is Daily Management System (DMS)and how does it help manufacturers?
The Daily Management System (DMS), also known as Daily Direction Setting (DDS), is a framework for managing operational performance in manufacturing organizations. It is a structured approach to managing day-to-day operations that focuses on continuous improvement and waste reduction.
Read MoreEpisode 5: Eliminate Admin Time and Automate Tracking With Digital BBSOs with Steve McBride, CEO & Founder of Weever
In this episode of Digitization Mavericks, we are joined by Steve McBride, Weever’s very own CEO and Founder. Steve is a manufacturing veteran with more than 30 years of industry experience and has worked with manufacturers across every industry.
Read MoreBetter Continuous Improvement Data through Digital Transformation
Juan is a process engineer with a decade of experience helping CPG manufacturers implement continuous improvement processes. In his latest role he found himself working at a small division of a large national food manufacturer that was just taking its first steps towards lean manufacturing and operational excellence.
Read MoreThe Importance of Cybersecurity in Protecting Manufacturing Systems
As manufacturing systems have adopted more remote measures over the last two years, the industry has become fodder for cybercriminals. In 2021, the manufacturing industry became the second most targeted just behind finance and insurance. This represents a 300% increase in a single year.
Read MoreEpisode 0: Effective Digital Workflows and Automation Don’t Exist Without People
In this episode, we feature a special interview between one of our podcast producers and the host of the Digitization Mavericks podcast. Now the current Sales Director at Weever, Chris shares details about his roots in manufacturing and his interests in technology beginning at a young age.
Read MoreWhy digitize your paper-based operations?
Weever specializes in digitizing operational workflows. We work with organizations of all shapes and sizes who are frustrated with paper forms and spreadsheets. They want to save money, become more efficient, and spend their time more wisely.
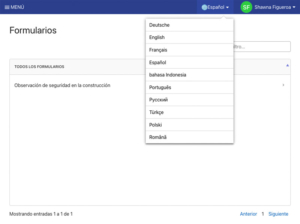
Read MoreWhat is Software Localization?
What is Software Localization? Andy Pritchard | April 6, 2022 | 5 min read What is Software Localization? Localization in software refers to an application changing state based on the language settings selected by the user. Localization considerations can include currency denominations and all “local aspects” of a culture or geographic area that influence…
Read MoreWhat is Root Cause Analysis and how does software help?
What is Root Cause Analysis and how does software help? Andy Pritchard | March 24, 2022 | 5 min read Root Cause Analysis (RCA) is a process that is usually required as part of other larger processes. Whether you are performing a CAPA (Corrective and Preventive Action) for a product defect or an Incident…
Read More